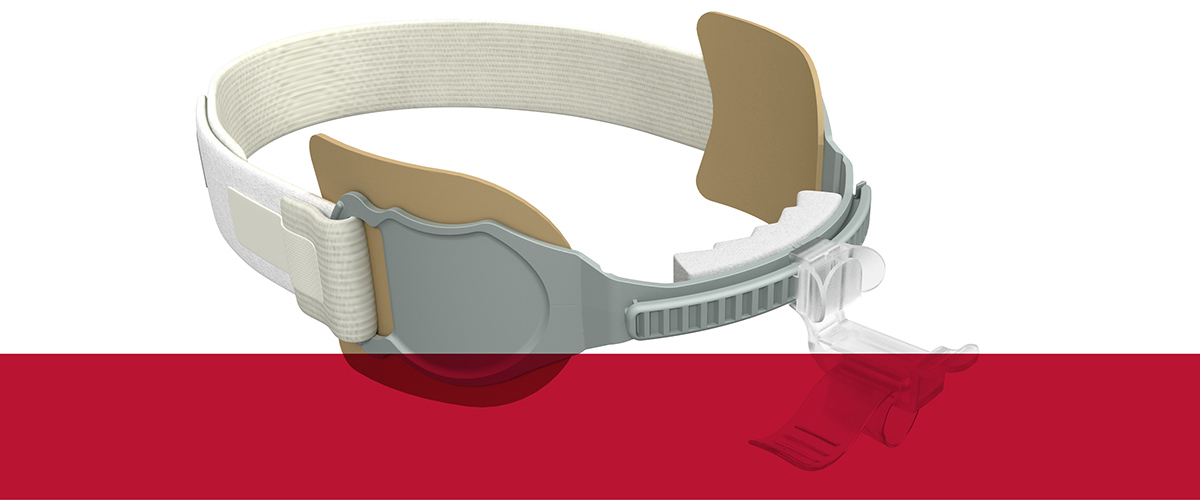
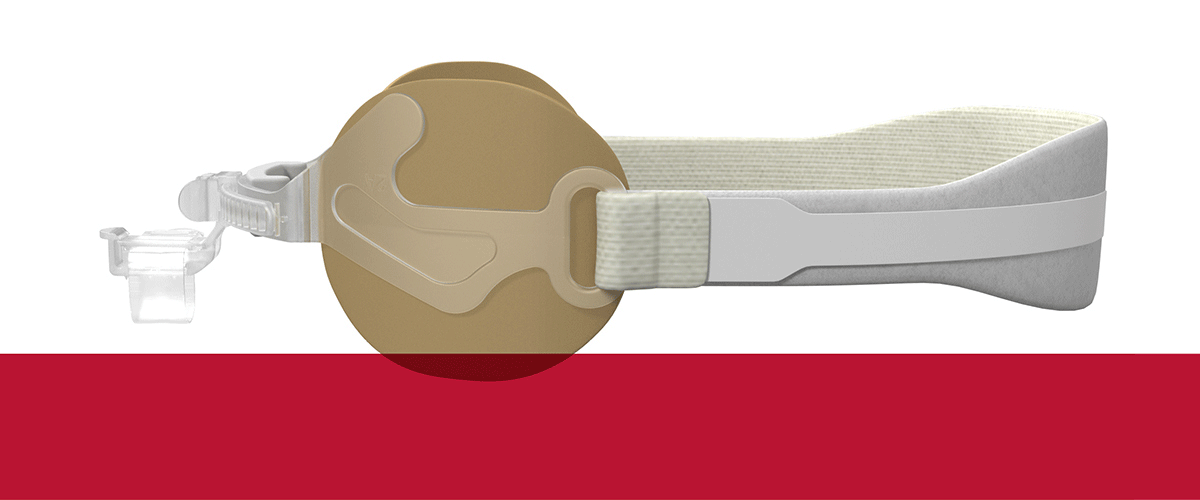
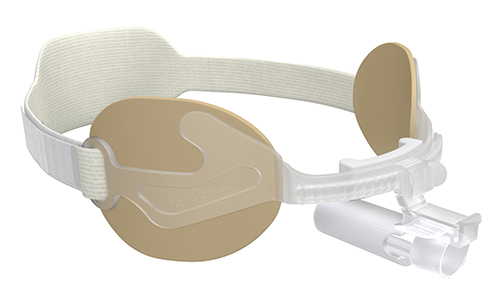
Our Family of AnchorFast Oral Endotracheal Tube Fasteners
Innovation and choices from your trusted brand
Every patient dependent on a ventilator for respiration is vulnerable. Intubated individuals face risks – such ventilator-associated events, oral pressure ulcers, and unexpected tube occlusions – that can result in longer lengths of stay and increased hospital costs.1
Our AnchorFast oral endotracheal tube fasteners help you address intubated patient risks with confidence. Our products offer the right combination of features for your patient’s needs, and are designed to provide many clinical benefits, including:
- Easing access to the oral cavity to help maximize oral care
- Helping prevent the formation of lip ulcers as part of a multi-faceted approach2,3
- Helping prevent tube occlusion with an integrated tube protection sleeve*
*AnchorFast Guard and AnchorFast Guard Select only
AnchorFast oral endotracheal tube fasteners products are used in thousands of hospitals around the world, and are the best-selling endotracheal tube securement devices on the market. At Hollister, customer feedback drives ongoing innovation, and our AnchorFast oral endotracheal tube fastener product line is continuing to evolve to meet your needs.
Click the tabs below to learn more about our product portfolio:
The AnchorFast SlimFit oral endotracheal tube fastener provides a convenient means to hold an oral endotracheal (ET) tube securely in place without the use of adhesive tape. It also is designed with the intent to accommodate smaller faces.
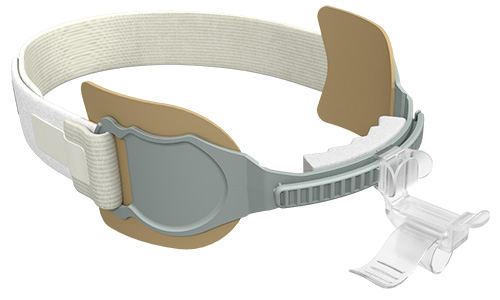

The AnchorFast oral endotracheal tube fastener, our trusted securement system, combines a number of features that make it the right choice. It is designed to ease access to the oral cavity to help optimise oral care.
The AnchorFast Guard oral endotracheal tube fastener is the right choice for critical care teams who want an extra measure of protection for their patients. With the addition of integrated tube protection to our trusted securement system, the AnchorFast Guard fastener can help to bring a new level of confidence to the ICU.
REFERENCES:
- Restrepo M, et al. “Economic Burden of Ventilator-Associated Pneumonia Based on Total Resource Utilization.” Infection Control and Hospital Epidemiology, 2010; 31(5):509-515.
- Miller, Kenneth, et al. “A Utilization of AnchorFast Oral Endotracheal Tube Fastener to Reduce the Incidence of Lip Ulcers.” Presented at The 55th International Respiratory Congress. 5-8 December 2009, San Antonio, Texas USA.
- Veneman, Wade, et al. “An Alternative Approach to Prevent Lip Pressure Ulcers in the Intubated Patient.” Presented at the Symposium on Advanced Wound Care. 17-20 April 2010, Orlando, Florida USA.
- Data on file. Clinical Study 5855-I, Multiple ICU settings, n= 30 patients using ET tubes with integrated subglottic suction capability
USA Rx Only. Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician or other healthcare practitioner licensed under state law to order this product. Prior to use, be sure to read the Instructions for Use for information regarding Intended Use, Contraindications, Warnings, Precautions, and Instructions.